At the core of life on Earth lies an intricate dance of electrons, orchestrated through the process of photosynthesis in plants and certain bacteria. This natural process, which transforms sunlight into chemical energy, finds its technological counterpart in photovoltaic systems where light is converted into electrical energy. Both phenomena hinge on the movement of electrons and charge transfer at the molecular level. Understanding the intricacies of these processes reveals not only how we can harness energy more efficiently but also sheds light on fundamental chemical dynamics.
In essence, the crux of this investigation lies in the ultrafast phenomena triggered by the absorption of light. When molecules interact with light, they experience instantaneous redistributions of electronic density. This crucial behavior is governed by quantum mechanics and points to the underlying molecular dynamics that define charge transfer. However, comprehending the details of these rapid events poses a significant challenge, especially regarding measurement techniques that can capture the fast pace of electron flow.
The quest for deeper knowledge regarding electron dynamics has fostered advancements in experimental techniques, particularly the use of ultrashort ultraviolet pulses produced by high-order harmonics or free-electron lasers. These sophisticated tools enable researchers to initiate and observe molecular reactions at unprecedented temporal resolutions, ranging from femtoseconds (10^-15 seconds) to attoseconds (10^-18 seconds). Despite remarkable progress in the field, many mysteries remain, especially concerning the early electron transfer stages following photoionization.
A recent breakthrough in this area stems from a collaborative effort between leading research institutions, including Politecnico di Milano and various institutes in Madrid. Their study, published in Nature Chemistry, reveals new dimensions of molecular dynamics through the application of attosecond extreme-ultraviolet (XUV) pulses. This groundbreaking work offers a pathway to unravel the interactions between electrons and nuclei in donor-acceptor systems, demystifying fundamental chemical processes.
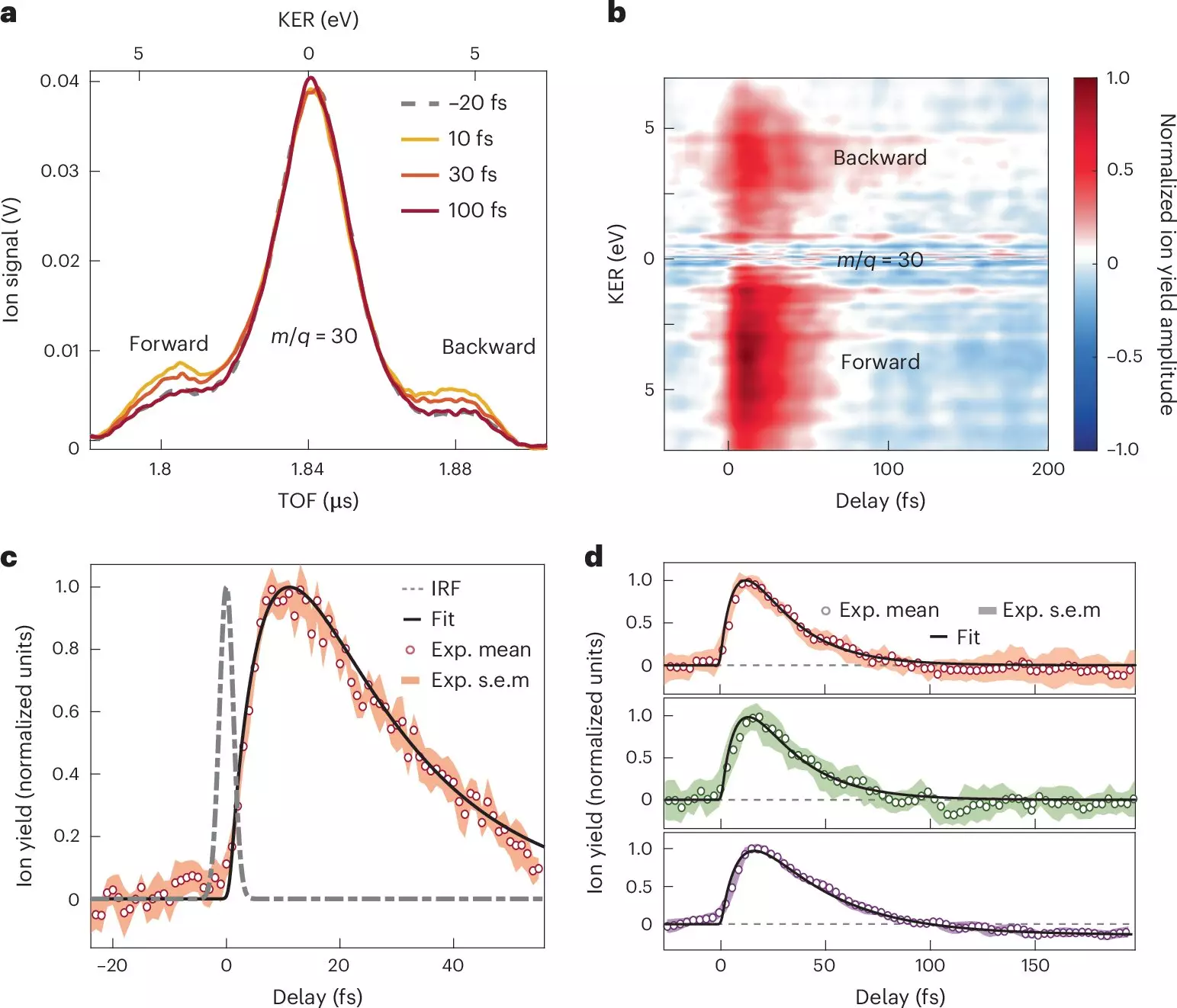
The research team, employing a combination of sophisticated methodologies such as attosecond XUV-pump and few-femtosecond infrared-probe spectroscopy, made significant strides in observing and analyzing charge transfer in molecular systems. By meticulously exposing nitroaniline molecules to these attosecond pulses, the study achieved previously unattainable precision in capturing the initial moments of charge transfer.
One of the pivotal insights from this research is the rapidity of electron transfer from the amino group, which occurs in less than 10 femtoseconds. This swift movement is facilitated by a synchronized dance of nuclei and electrons, highlighting the importance of electron-nuclear coupling in determining reaction pathways. The subsequent relaxation phase, which unfolds within a sub-30-femtosecond timescale, reveals how nuclear wave packets disperse within the excited electronic states of the molecular cation—underlining the complex interplay of structural and electronic changes during these rapid processes.
Rethinking Traditional Models of Charge Migration
This cutting-edge research not only broadens the understanding of molecular dynamics but fundamentally challenges existing models of charge migration in organic molecules. By detailing the timing and structural evolution during charge transfer, the study equips scientists with a richer vocabulary and framework for describing these phenomena.
Furthermore, the findings contribute to a deeper understanding of charge migration in donor-acceptor systems, particularly how electronic and structural transformations interrelate during photoinduced processes. Such insights not only enrich theoretical models but may also inform future experimental designs.
The implications of this study extend beyond academic understanding and enter practical realms, particularly in the fields of chemistry, materials science, and energy research. The insights gained through attosecond science may pave the way for innovations in organic electronics, photovoltaics, and perhaps even quantum computing, where the manipulation of electronic states is paramount.
As researchers continue to unravel the complexities of molecular dynamics through advanced time-resolved techniques, we stand on the precipice of a new era in understanding charge transfer processes. This knowledge not only elevates our comprehension of fundamental chemical principles but also catalyzes future advancements in technologies that can enable more efficient energy solutions and explore the quantum landscape. The journey into the realm of attosecond dynamics is just beginning, and its trajectory promises to reshape the scientific landscape for years to come.

Leave a Reply
You must be logged in to post a comment.