The quest for sustainable energy solutions has taken a noteworthy turn with research from the University of Michigan, where scientists have pioneered an artificial photosynthesis system capable of converting carbon dioxide (CO2) into valuable hydrocarbons. This process, which chains carbon atoms together to synthesize ethylene, has demonstrated unprecedented performance, heralding a promising pathway toward utilizing waste CO2 in the production of eco-friendly fuels and materials. Ethylene is not only the world’s most produced organic compound, primarily used in the plastics industry, but this innovative approach can significantly reshape how it is manufactured, promoting a circular economy by reducing reliance on fossil fuels.
The conventional production of ethylene generally involves high-temperature and high-pressure processes reliant on oil and gas, resulting in substantial CO2 emissions. The University of Michigan team sought to transform this problematic synthesis by employing a process driven by solar energy. Their system reportedly achieves a remarkable efficiency rate that is approximately five to six times better than current methods of solar-driven carbon dioxide reduction to ethylene. This leap in performance opens up a panoramic view of industrial possibilities, significantly mitigating environmental impacts connected with traditional hydrocarbon synthesis.
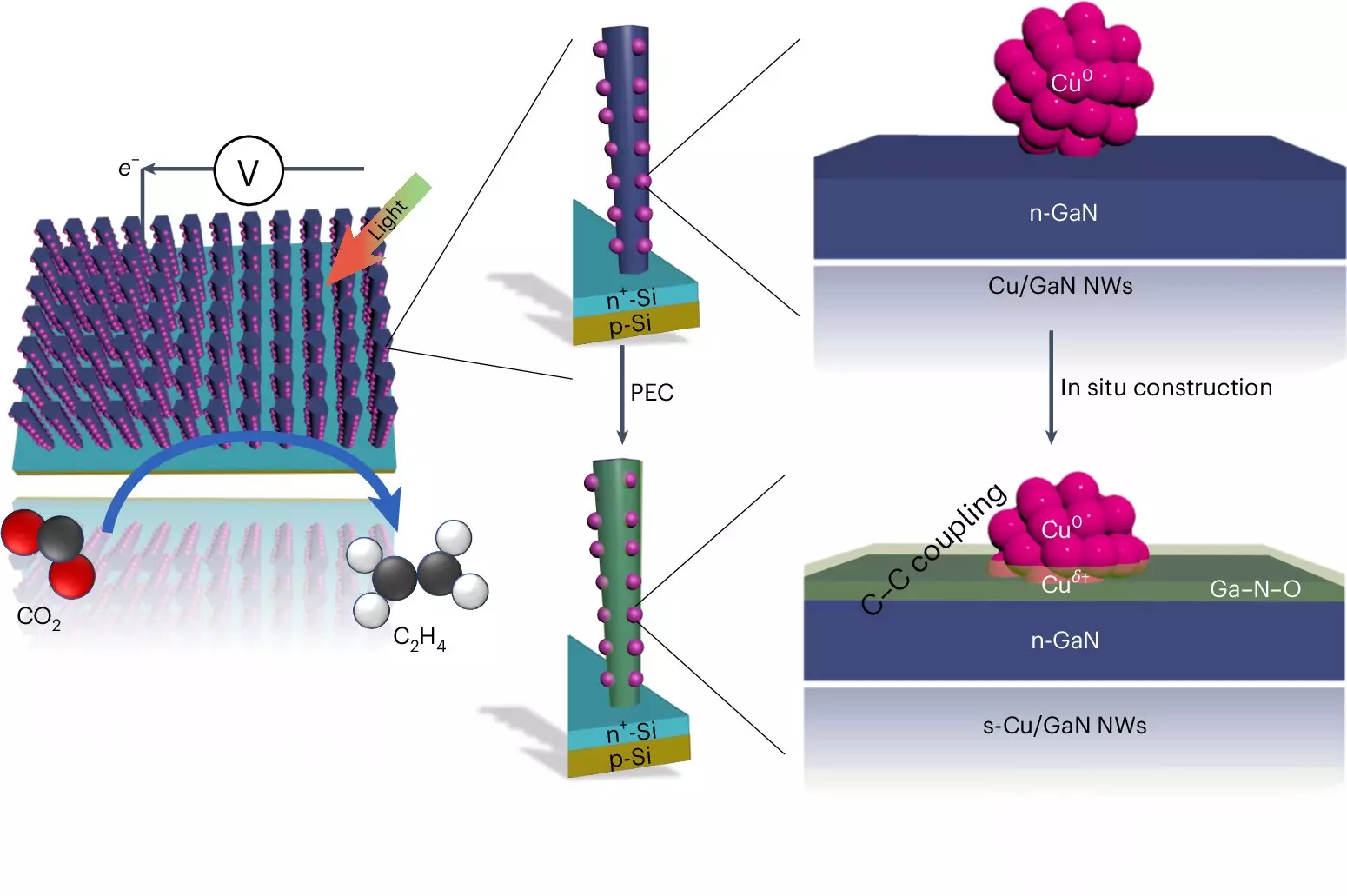
At the heart of this innovative system lies a sophisticated mechanism involving advanced materials. The apparatus utilizes two types of semiconductors: gallium nitride nanowires and a silicon substrate, both of which absorb sunlight effectively. The nanowires, measuring just a few hundred atoms in diameter, are critical for the catalytic process, converting water and CO2 into ethylene. It operates through a two-pronged approach where sunlight frees electrons, while simultaneously splitting water molecules to produce hydrogen. As part of this reaction, copper clusters serve as catalysts, enabling the transformation of captured carbon dioxide into carbon monoxide—an essential intermediate in hydrocarbon synthesis.
This process, detailed by Professor Zetian Mi, illustrates the unique interplay between the gallium nitride and copper, where there is a synergistic relationship enhancing both hydrogen retention and carbon conversion. The ability of gallium nitride to absorb oxygen and convert it into gallium nitride oxide not only provides a self-healing mechanism for the catalyst but also ensures sustained reaction efficiency.
One of the standout features of this technology is its impressive longevity. While comparable systems might only operate effectively for several hours, the Michigan team’s invention sustained its operation for 116 hours without a drop in efficiency, with additional tests indicating that similar systems could run for up to 3,000 hours. This durability addresses one of the primary concerns in the fields of catalysis and energy conversion: maintaining operational stability over long periods.
The potential applications for this technology extend beyond ethylene production. The researchers have their sights set on creating longer-chain hydrocarbons that can be transformed into liquid fuels—a critical goal that aligns with the increasing need for sustainable energy sources in transportation and industries. Assistant Research Scientist Bingxing Zhang expressed aspirations to produce multi-carbon compounds like propanol, aiming to expand the toolkit for sustainable fuel options.
Despite these promising advancements, the journey ahead is fraught with challenges. The research community must continue exploring ways to maximize the production of longer hydrocarbons while ensuring that the reactions remain efficient and sustainable. As the team at the University of Michigan pushes the boundaries of what is possible with artificial photosynthesis, their ongoing work will focus on refining these processes, enhancing output, and minimizing any residual environmental impact.
As the urgency of climate change weighs heavily on global leaders and researchers alike, breakthroughs in artificial photosynthesis like this one are vital. They not only represent technological innovations but also symbolize the potential for sustainable practices that could reshape our interaction with energy and resources. The implications of successfully harnessing CO2 for fuel production could reverberate through multiple sectors, fostering a transition to a circular economy that prioritizes environmental stewardship while addressing the energy demands of the future.

Leave a Reply
You must be logged in to post a comment.