In a remarkable feat, researchers at the Vienna University of Technology (TU Wien) have managed to produce laser-synchronized ion pulses lasting under 500 picoseconds, paving the way for unprecedented studies of chemical processes on material surfaces. Their findings were published in the prestigious journal, Physical Review Research. This breakthrough has the potential to transform our understanding of rapid chemical reactions by allowing scientists to capture and analyze these processes as they unfold in real time.
To describe phenomena occurring in the microscopic world, traditional methods often fall short. The velocity of the events typically far exceeds the capabilities of conventional observation techniques—similar to needing a camera with ultra-fast shutter speeds to effectively capture motion. Here, the concept of time resolution becomes crucial. Ultra-short laser pulses have been instrumental in providing insights into atomic-level phenomena. However, a novel dimension has emerged in the form of ion pulses, which serve as powerful tools to extend our observational reach into chemical reactions.
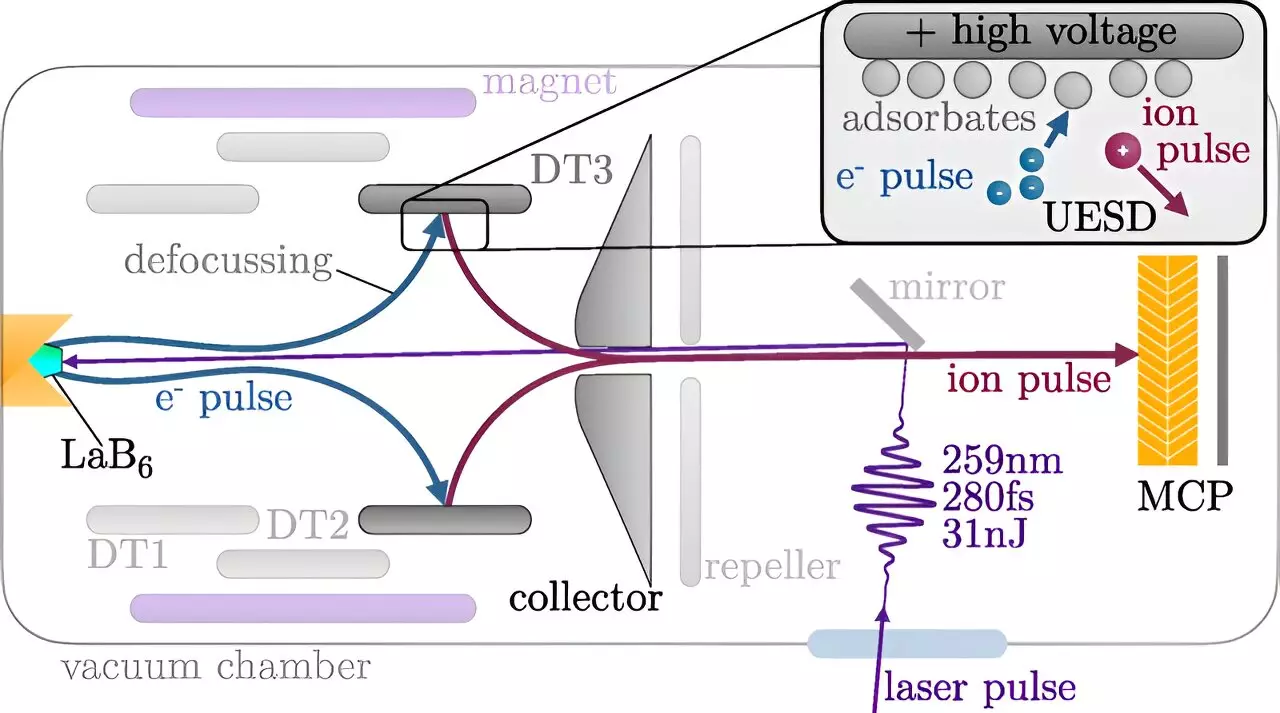
Historically, ion beams have stood as valuable assets in material science for analyzing structures, cleaning surfaces, and modifying materials. Yet, these applications have usually focused on examining outcomes rather than the kinetics of processes. As described by Professor Richard Wilhelm from the Institute of Applied Physics at TU Wien, in conventional ion beam processes, the results are observed after the impact of the ions—the intermediate transformations remain a mystery. With the advent of laser-synchronized ion pulses, this paradigm is shifting dramatically.
The core mechanical process behind the generation of these ultrashort ion pulses begins with a laser pulse directed at a cathode, initiating the emission of electrons. These electrons are subsequently accelerated to collide with a stainless steel target coated with various atoms, such as hydrogen and oxygen. When the electrons strike, they dislodge these surface atoms, resulting in a mix of ionic and neutral particles. The ability to harness electric fields allows the researchers to selectively channel these expelled atoms—transforming them into targeted ion pulses ready for material surface analysis.
The engineering intricacies of this method are commendable; the system can control the timing of ion generation and delivery with precision. This precise control means that researchers can time the ion impacts to coincide with specific chemical reactions powered by laser activation, thus enabling the monitoring of reactions on an incredibly fine temporal scale.
While protons, the simplest form of ions, have been the initial focus, the methodology is versatile enough to accommodate a range of ion types. The length of the ion pulses is largely dictated by the type of atoms that coat the stainless steel target, indicating a manipulatable dimension within this research. Future experiments might introduce carbon or oxygen ions into the mix, offering varied analyses that could deepen our understanding of chemical dynamics. The researchers demonstrate an intriguing breadth, suggesting electrically neutral and negatively charged ions could also be utilized, expanding the analytical toolkit available for surface science.
Excitingly, there are already proposals to minimize the duration of these ion pulses even further. This could be achieved through specially-shaped alternating electromagnetic fields that would allow varied manipulation of ion velocities in a pulse. Such enhancements could propel our understanding of ultrafast processes to new heights, revealing intricacies of dynamics previously deemed inaccessible. As Wilhelm notes, this has the potential to merge with current ultrafast electron microscopy techniques, enhancing the capabilities available to physicists and chemists alike.
In sum, the research at TU Wien signifies a major leap forward in our ability to monitor and analyze chemical reactions on material surfaces in real time. By combining laser technology with precision ion beam techniques, scientists are poised to uncover the mechanisms of fast chemical processes with unprecedented clarity. As the layers of complexity behind chemical behaviors are peeled back, we may well be on the brink of transformative advancements in both theoretical and applied sciences, charting new territories within the realms of physics and chemistry alike.

Leave a Reply
You must be logged in to post a comment.