Lithium-metal batteries have long been hailed as the future of battery technology due to their potential for significantly higher energy densities than lithium-ion batteries. However, these cells have historically been plagued by limitations, most notably a short lifespan. Researchers at the University of Science and Technology of China and other institutes have recently introduced a new electrolyte design that could revolutionize the development of lithium-metal batteries.
The Challenge of Lifespan
One of the major challenges facing lithium-metal batteries is their limited cycle life, typically around 50 cycles compared to the 1,000 cycles of commercial lithium-ion batteries. The growth of lithium dendrites, high reactivity of lithium-metal, and high-voltage transition metal cathodes contribute to constant degradation of the electrolyte, hindering the performance of these batteries. Despite extensive research efforts, lithium-metal batteries have yet to achieve the desired energy density and cycle life.
Approximately five years ago, researchers developed an electrolyte that could stabilize the interfaces between the electrolyte and electrodes in lithium-metal battery cells. This design aimed to suppress electrolyte degradation and improve battery performance. By focusing on microscopic physicochemical processes inside lithium-metal batteries, the researchers were able to enhance the stability of these batteries, laying the groundwork for further advancements.
Unique Solvation Structure
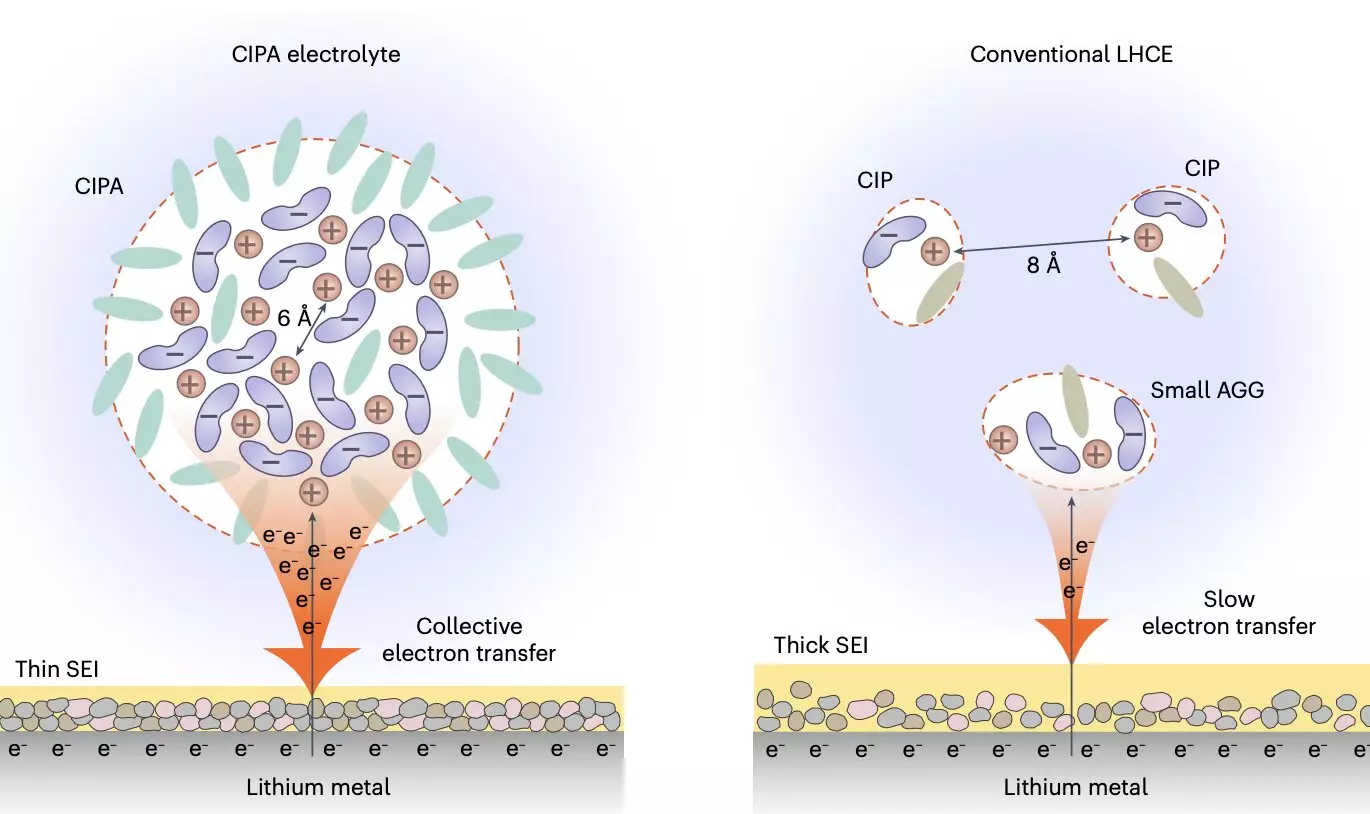
The recent study led by Prof. Jiao and her colleagues focused on the solvation structure of the electrolyte at the mesoscopic level. Their innovative design centered on ion pairs within the electrolyte’s aggregate structure, resulting in the formation of compact ion-pair aggregates (CIPA). This unique solvation structure promotes collective reduction on the lithium-metal anode, leading to the formation of a thin and stable solid electrolyte interface (SEI) that suppresses electrolyte decomposition.
Enhanced Battery Performance
The new electrolyte design not only improves the stability of the lithium-metal anode but also enhances the oxidative stability and suppresses the dissolution of transition metal elements from the cathode. This dual effect results in stable cycling for a prolonged number of cycles. By introducing a new class of electrolytes with a mesoscopic solvation structure, the research team has paved the way for advancements in the design of lithium-metal batteries.
The potential of the newly designed electrolyte was demonstrated in a 500 Wh/kg lithium-metal pouch cell, which retained 91% of its energy after 130 cycles. The researchers are now focused on further extending the cycle life of these cells to over 1,000 cycles. Additionally, efforts are underway to explore new battery systems with even higher energy densities, such as achieving 600 Wh/kg with 100-200 cycles. The future of lithium-metal batteries looks promising with ongoing research and development in electrolyte design and battery technology.
The recent advancements in electrolyte design for lithium-metal batteries offer a glimpse into the future of high-energy-density battery technology. With innovative approaches to stabilizing electrode-electrolyte interfaces and enhancing battery performance, researchers are inching closer to realizing the full potential of lithium-metal batteries. As efforts continue to improve cycle life, energy density, and overall performance, lithium-metal batteries may soon become the standard for next-generation battery technology.

Leave a Reply
You must be logged in to post a comment.