In the realm of physics, understanding the intricate interactions between different substances is essential for predicting the behavior of complex systems. Classical mixture theory offers a framework to analyze these interactions, particularly in systems where two or more constituents coexist, such as the phenomenon of phase separation. Notably, these principles have been applied beyond traditional physics realms, finding relevance in biological systems where protein compartmentalization plays a crucial role. Recent research from São Paulo State University (UNESP) opens new avenues in this interdisciplinary approach, linking concepts from condensed matter physics to cellular processes.
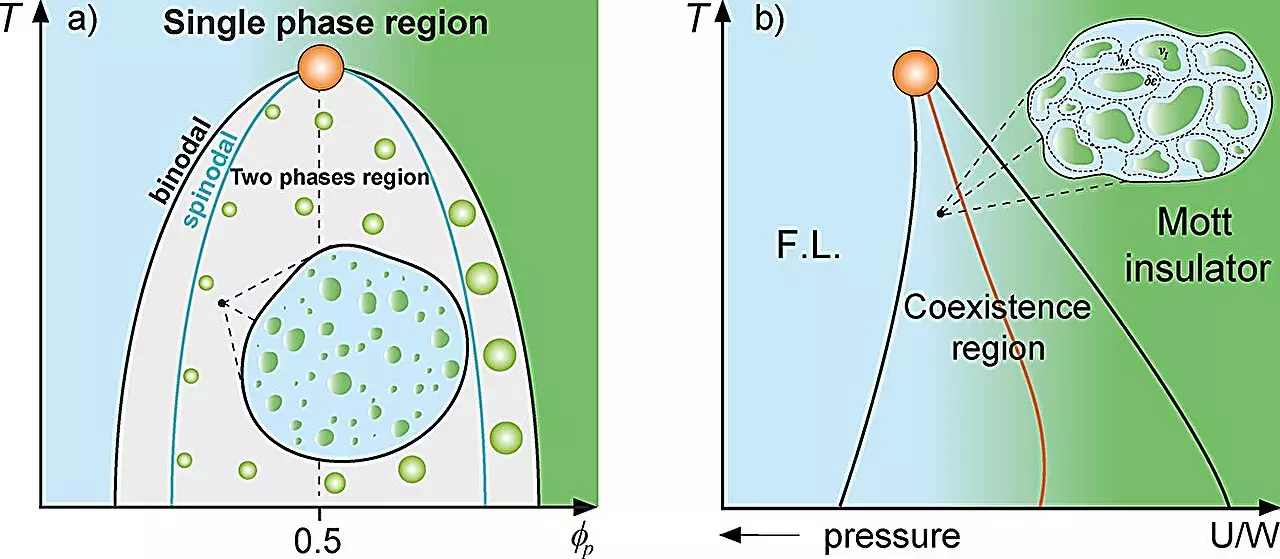
The Griffiths phase, initially described in the context of magnetism, refers to regions within a material that can exhibit distinct magnetization, despite being embedded in a non-magnetized matrix. These ‘rare regions’ serve as critical points where the dynamics of the overall system exhibit considerable variation. This concept has been established through previous studies exploring electronic properties at the Mott metal-insulator transition. The application of this concept to cellular biology by UNESP researchers suggests that similar rare regions can manifest within cellular environments, influencing protein behavior and, consequently, cell function.
Under the leadership of Professor Mariano de Souza, the recent study delves into the dynamics of protein droplets formed during liquid-liquid phase separation within cells. These protein droplets, akin to the rare regions observed in magnetic materials, provide a framework for understanding how cellular environments adapt and react to variations in protein concentration. By utilizing advanced thermodynamic models, such as the Grüneisen parameter and the Flory-Huggins model, the researchers highlight the dramatic reduction in cellular dynamics that occurs near the phase separation thresholds.
This reduction in dynamics is not merely a passive phenomenon; it has profound implications for cellular function. By delineating the conditions under which proteins aggregate into droplets, the research provides insights into how such processes might optimize gene expression by minimizing stochastic fluctuations—the randomness that can lead to erratic cellular behavior.
One particularly compelling aspect of this study is its connection to theories surrounding the origin of life. Drawing on the early 20th-century theories posited by Aleksandr Oparin, the authors suggest that coacervates—organic droplets in aqueous solutions—exhibit slow dynamics that may have favored the survival and evolution of primordial organisms. This perspective reinforces the idea that specific physical conditions can significantly influence biological emergence and complexity. The implications of these findings could reshape our understanding of life’s origins, suggesting that physical states, such as those described in the Griffiths-like cellular phase, could play foundational roles in early biological development.
An intriguing point raised in the research is the concept of chirality—the phenomenon where certain molecules cannot be superimposed on their mirror images. Chirality is fundamental in biological systems, where homochirality, or the predominance of one chiral form, is crucial for molecular compatibility and function. The researchers argue that the reduction of dynamic fluctuations in a Griffiths-like cellular phase may enhance the role of homochirality, thereby influencing evolutionary trajectories. This provides a novel intersection between physics and biochemistry, implicating physical states in critical biological processes.
Beyond understanding cellular function and origins, the study has significant implications for disease research. The role of liquid-liquid phase separation in various diseases, including cancer, neurodegenerative disorders, and even viral infections like COVID-19, underscores the importance of these findings in therapeutic contexts. Compartmentalization of proteins can affect their functionality, which can, in turn, lead to enhanced understanding and potential treatments for diseases characterized by aberrant protein aggregation.
The researchers emphasize that understanding the dynamics of these cellular phases can be pivotal in developing new strategies for managing diseases and improving treatment outcomes. The Griffiths-like cellular phase may indeed serve as a double-edged sword; while it potentially enhances cellular organization and efficiency, it may also contribute to pathological states depending on the context.
The study conducted by de Souza and his team epitomizes the growing trend of interdisciplinary research, where physics meets biology in a quest to unravel the complex mechanisms that underpin life. By employing theoretical frameworks from condensed matter physics to unravel cellular processes, the researchers are not just augmenting our understanding of biology but potentially paving the way for innovative therapeutic interventions. As science continues to evolve, such interdisciplinary approaches will be critical in addressing the multifaceted challenges presented by biological systems and diseases.

Leave a Reply
You must be logged in to post a comment.